|
Taxol rival gets go-ahead
|
 |
September 15, 2000: 3:35 p.m. ET
IVAX's anti-cancer drug receives final FDA approval, but no launch date set
|
NEW YORK (CNNfn) - Drug maker IVAX Corp. announced Friday it has won U.S. regulatory approval to sell a generic version of Taxol, Bristol-Myers Squibb Co.'s popular drug to fight ovarian and breast cancer.
Miami-based IVAX has been battling the New York-based pharmaceutical company in a lengthy patent fight over the right to introduce a generic form of Taxol, the world's best-selling cancer treatment with about $1 billion in annual sales. IVAX's copycat version would be the first generic competitor to the drug.
The Food and Drug Administration approval, which had been expected, clears the way for the Miami-based generic drug maker to begin marketing of paclitaxel, the chemical name for the drug, in the United States. The generic version already is available in Europe and Canada.
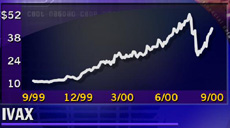 But IVAX (IVX: Research, Estimates), still fighting Bristol-Myers (BMY: Research, Estimates) in court, declined to discuss when the product would go on the market. But IVAX (IVX: Research, Estimates), still fighting Bristol-Myers (BMY: Research, Estimates) in court, declined to discuss when the product would go on the market.
"At this moment, we are not commenting into our launching of the product," Vice President and Treasurer Jordan Siegel told CNNfn.com.
The FDA approval gives IVAX exclusive rights to market a generic version for 180 days. IVAX's drug could generate more than $100 million in sales in its first full year on the market, according to analysts' forecasts.
IVAX has accused Bristol-Myers of attempting to delay introduction of its cheaper product. Earlier this week, a federal appeals court in California denied a Bristol-Myers' motion that would have blocked approval of IVAX's drug.
IVAX shares jumped on the FDA approval news, gaining $3 to $44.25 in afternoon trading. Bristol-Myers stock added 56 cents to $53.88. 
-- from staff and wire reports
|
|
|
|
|
 |

|

