|
New tricks for old drugs?
|
 |
November 8, 2000: 9:24 a.m. ET
Pharmaceutical makers study how to extend the life of blockbuster drugs
By Staff Writer Martha Slud
|
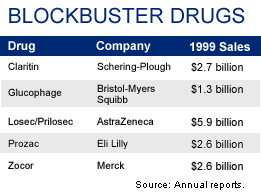
NEW YORK (CNNfn) - Over the past decade, Schering-Plough Corp.'s Claritin brand of allergy drugs have reaped about $9 billion in total worldwide revenue. It's no wonder, then, that the company would do whatever it takes to keep the franchise on its best-selling medicine going and going and going.
Schering-Plough (SGP: Research, Estimates) -- like many other drug makers -- is becoming more creative and aggressive in crafting new uses for blockbuster medications in the most lucrative markets, such as allergies, heart disease and depression. For Schering-Plough, the introduction of a follow-up allergy product could not come a moment too soon -- the company might lose patent exclusivity on its cash cow Claritin as early as 2002 and it is eager to introduce a "new and improved" antihistamine that could discourage patients from turning to cheaper, generic drugs when they become available.
"Claritin is our No. 1 product. We are very focused on refining Claritin or coming up with what might be seen as the second- or third-generation Claritin down the road," said company spokesman Robert Consalvo.
 Madison, N.J.-based Schering-Plough is awaiting U.S. Food and Drug Administration approval for a revamped version of Claritin. The new version, known technically as desloratadine but often dubbed "Son of Claritin" in pharmaceutical circles, was developed in conjunction with Marlborough, Mass.-based biotechnology firm Sepracor Inc. (SEPR: Research, Estimates). Sepracor would receive royalty payments if the once-a-day treatment for hay fever goes on the market. Madison, N.J.-based Schering-Plough is awaiting U.S. Food and Drug Administration approval for a revamped version of Claritin. The new version, known technically as desloratadine but often dubbed "Son of Claritin" in pharmaceutical circles, was developed in conjunction with Marlborough, Mass.-based biotechnology firm Sepracor Inc. (SEPR: Research, Estimates). Sepracor would receive royalty payments if the once-a-day treatment for hay fever goes on the market.
Meanwhile, Schering-Plough has paired with larger rival Merck & Co. (MRK: Research, Estimates) on a proposed new allergy treatment that would combine Claritin with Merck's fast-growing asthma drug, Singulair. The experimental treatment is undergoing advanced, Phase III clinical trials.
Schering-Plough and Whitehouse Station, N.J.-based Merck also are developing a combination heart disease treatment linking Merck's blockbuster cholesterol-lowering treatment, Zocor, with an experimental compound developed by Schering-Plough designed to block cholesterol absorption.
Pharmaceutical companies are developing new versions of successful drugs -- either chemical cousins that may be more potent or produce fewer side effects than the original, or combination therapies that use their product in conjunction with another compound to treat a different type of illness or which offer more effective treatment. The new products can't prevent the introduction of generic competition to the original brands, but they can help the companies retain patients who otherwise might choose the cheaper generic version made by someone else.
Pharmaceutical analyst Mariola Haggar, of Concord Haggar Partners, says the strategy is simple: Drug companies can get more bang for their buck by finding new uses for tried-and-true products.
"It actually can be a very strong protection against the patent expiration, especially if you can sell the drugs as a combination pill or a combination regiment," she said.
| |

|
|
| |
|
|
| |
"I think the strategy first and foremost will only work if the new combination or the new delivery form really does provide an advantage."
|
|
| |
|
|
| |

|
|
| |
|
|
| |
Mariola Haggar
Haggar Concord Partners |
|
She said companies have already done the costly research and clinical testing to make sure the products are safe and effective, legwork that would be much more expensive and risky if it had to be repeated for a brand new medication.
However, unless the new drug represents a significant advance, pharmaceutical companies will have a tough time persuading doctors to prescribe a new version with basically the same active ingredients rather than a cheaper generic version of the original product, analysts say. They say that Schering-Plough, for example, likely will have to conduct a costly promotion campaign to persuade patients to switch products.
"I think the strategy first and foremost will only work if the new combination or the new delivery form really does provide an advantage," said Haggar. "If there is no advantage, the strategy will not work. Once the patent expires, it (the drug) becomes so cheap in its generic form. Why would anyone use an expensive brand drug -- even if it is combined with something else -- if there is no advantage."
Soaring R&D costs
For drug companies, the strategy can potentially save a lot of money at a time when pipelines are running thin and research and development costs are soaring. The average new drug takes about eight-to-10 years to develop and an estimated $500 million dollars to develop. Failure rates are high.
Meanwhile, pharmaceutical companies are spending heavily on costly biotechnology initiatives capitalizing on the human genome project. Drug companies are investing in pricey drug development technology, and signing more pacts with biotech firms to identify potential drug targets for scores of diseases.
Analysts say that's why drug companies are so keen to keep their successful franchises going -- introducing next-generation products whenever possible that are touted as improvements on the original but that require much less work than a new product developed from scratch.
Consalvo, of Schering-Plough, says that while drug development using existing medications is still a long and arduous process, such collaborative projects do give researchers an advantage because they are working with established products that are already known by doctors and in the marketplace.
"We know we have a product that is very accepted," he said. "Instead of going back to ground zero, sometimes it makes more sense to partner together."
A widespread practice
Many other drug makers also are tinkering with existing treatments to cook up new versions. New York-based Bristol-Myers Squibb Co. (BMY: Research, Estimates), for example, recently introduced a new version of its hot-selling diabetes treatment Glucophage. The new version, Glucovance, is billed as an improved version of Glucophage. It went on the market in August.
AstraZeneca PLC (AZN: Research, Estimates), meanwhile, has introduced a successor to its blockbuster ulcer drug, Prilosec, which has already lost patent exclusivity in several European markets. (The drug is known as Losec outside of the United States.) The new version, Nexium, recently went on the market in Europe.
In the biotechnology area, Biogen Inc. (BGEN: Research, Estimates) has teamed up with Irish drug company Elan Corp. (ELN: Research, Estimates) to develop a new treatment for multiple sclerosis and Crohn's disease -- a drug that could be used alone or in conjunction with Biogen's blockbuster MS drug Avonex.
A new market for Avonex could be crucial for Biogen's future revenue growth. Sales of Avonex accounted for about 78 percent of the Cambridge, Mass.-based company's total revenue last year.
But not all such projects are successful. Eli Lilly and Co. (LLY: Research, Estimates) recently dropped a development project for a next-generation version of its antidepressant Prozac.
The Indianapolis-based company had licensed the isomer R-fluoxetine from Sepracor, the same company that played a role in developing the next-generation Claritin. But Lilly abandoned the project for the reformulated Prozac after several participants in clinical trials developed cardiac side effects from the experimental treatment. Sepracor shares slid about 40 percent when the news was announced last month.
Analysts say drug makers are likely to increase partnerships with one another as drug research becomes even more expensive and medical advances allow companies to develop new formulations of successful drugs relatively easily.
Consalvo said such partnerships between drug makers with expertise in specific areas can have the potential to bring products to the market sooner. Schering-Plough joined with Merck in developing its experimental cholesterol compound in part because Merck is one of the industry leaders in the category, he said.
"The intent here was to draw upon the expertise in both companies, which in theory, will prove to bring these products to market perhaps sooner than if either company had set out to do this on their own," he said. 
|
|
|
|
|
 |

|

